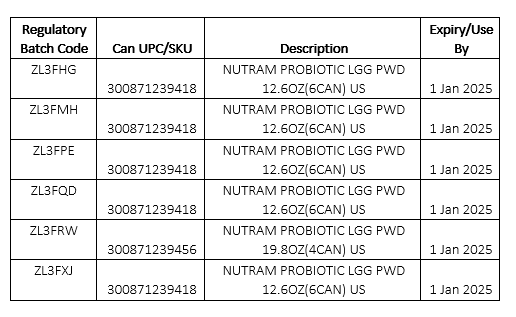
This is to inform you of a voluntary product recall involving six (6) select batches of Nutramigen Hypoallergenic Infant Formula Powder in 12.6 and 19.8 oz cans (product details below). This action was taken due to a possibility of cross-contamination with Cronobacter sakazakii. All product distributed went through extensive testing by Reckitt/Mead Johnson Nutrition and tested negative for the bacteria.
Cronobacter occurs naturally in the environment and can be found in or on household surfaces – including food preparation surfaces – dust, kitchen areas, food or even water. Although all infant formulas are produced under stringent hygienic conditions and comply with international microbiological specifications, the process of making powdered formulas cannot make them commercially sterile.
Cronobacter is a pathogenic bacteria that can cause illness, mainly in infants younger than two months old and those born premature, have weakened immune systems, or are of low birth weight. Cronobacter is naturally found in the environment and can survive in low-moisture, dry foods, such as powdered infant formula and milk, herbal teas, and starches. Symptoms related to infection in infants may include poor feeding, irritability, temperature changes, jaundice, grunting breaths, or abnormal body movements.
Products subject to this recall were manufactured in June 2023 and distributed primarily in June, July, and August 2023. Based on the limited availability of the remaining stock of this special infant formula, we do not expect the recalled product to be available on retail shelves. It is believed that much, if not all, of the products recalled have been consumed. No illnesses or adverse consumer reactions have been reported to date.

At Reckitt, we are committed to the highest level of quality and safety, and it is for this reason that we have taken this extraordinary measure. Reckitt/Mead Johnson Nutrition tested the batches in question and results were negative for Cronobacter and other bacteria. We believe this to be an isolated situation.
The health and safety of infants is our highest priority. All our products undergo rigorous and industry leading quality tests and checks to ensure that they meet or exceed all standards set by regulatory bodies, including the World Health Organization and the U.S. Food and Drug Administration. It is for this reason that we have confidence in the safety and quality of every infant formula we make.
Return Instructions
Immediately examine your inventory and quarantine product subject to this recall. In addition, if you have further distributed this product, please identify your customers, and notify them at once of this product recall. Your notification to your customers may be enhanced by including a copy of this recall notification letter. We request you complete the response form below and return to us as soon as possible.
Once you have quarantined impacted products, please follow the instructions below to return the product. Please note, only recalled lots will be credited.
This recall should be carried out to the consumer level. Your assistance is appreciated and necessary to prevent any adverse events associated with Cronobacter.
This recall is being made with the knowledge of the Food and Drug Administration.
VP Sales, Nutrition
Wendy Hosier
Senior Commercial Quality Manager
Reckitt – Health & Nutrition